UC San Francisco researchers have visualized the earliest stages of pregnancy in unprecedented detail in laboratory animals and human tissue using new laboratory imaging techniques that promise to enable rapid progress in understanding disorders of pregnancy and improving the success rate of in vitro fertilization technology.
In mice, the team visualized for the first time how the uterine lining changes shape at the time of implantation to make pockets for the early embryo to burrow into, and how uterine glands and their ducts – also visualized in their entirety here for the first time – reorient themselves towards the site of implantation, potentially to help nourish the embryo before its placenta and umbilical cord develop. The researchers also demonstrated the utility of their approach in studying the human uterus by visualizing the uterine glands in uterine tissue removed during hysterectomies.
The research — published online in its final version on December 13, 2016, in the journal Development — was led by Ripla Arora, PhD, a post-doctoral researcher in the laboratory of Diana Laird, PhD, an associate professor of obstetrics, gynecology and reproductive sciences at UCSF and senior author on the new paper.
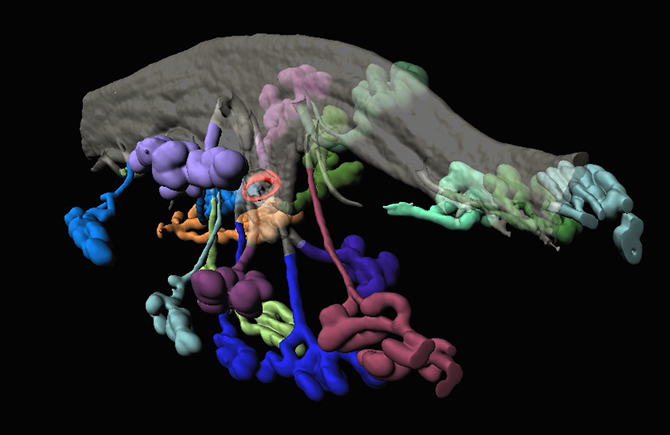
Blastocyst (red) in the uterine lumen (translucent grey) surrounded by branched uterine glands (colorful blobs) at the time of implantation in the mouse.
Newly revealed uterine glands and pockets in uterine lining may support the early embryo
The ability to grow an embryo in a dish from the time of fertilization until it is capable of implanting into the mother’s uterine lining has led to great advances in understanding processes crucial for an embryo’s survival in the weeks following fertilization. On the other hand, studies on how the mother’s uterine environment physically interacts with the embryo and contributes to its growth during this period have lagged, Laird says, in part because finding and studying the embryo when it first implants in the uterus was extremely difficult and time consuming with existing techniques.
To address this gap, the UCSF team applied a method for 3D imaging and analysis of the early mouse embryo in its intact uterine surroundings which they had previously developed to study the ovaries. The approach involves chemically “clearing” uterine tissue in the lab, and then using fluorescently tagged antibodies to highlight different components of the uterine lining. Imaging of the tissue and 3D rendering of the embryo and uterine glands relied on the high-end confocal microscopy core supported by the Center for Regenerative Medicine and the computational image analysis resources of the Biological Imaging Development Center run by UCSF’s Department of Pathology.
Using their new approach, the team observed that the uterine lining becomes extensively folded as it approaches its window of receptivity for an embryo to implant. The geometry of the folds in which the incoming embryos dwell is important, the team found, as genetic mutants with defects in implantation have improper patterns of folding.
The imaging technique also revealed the structure of elusive uterine glands that had long been hypothesized to nourish newly implanted embryos. The team observed for the first time that the uterine glands are connected to the uterine lining by narrow ducts and that the glands and ducts dramatically change their shape and orientation at the time of implantation: stretching out and reorienting towards the embryo like sunflowers facing the sun.
“You can tell where the embryo is right away by looking at the orientation of the glands,” Arora said. “This is something no one has ever been able to observe before. The fact that these glands — which other techniques always showed as sort of floating in space around the uterus — actually connect to the interior of the uterus and grow towards the implantation site suggests that they probably do secrete factors that prepare the uterine lining to accept the embryo and may support the embryo during its first few days.”
Technique aims to spur new advances in understanding earliest stages of pregnancy
The researchers also used their technique to make initial observations about the structure of uterine glands in human uterine tissue from the NIH UCSF Human Endometrial Tissue and DNA Bank, which contains samples of women undergoing endometrial biopsy or hysterectomy, directed by co-author Linda Giudice, MD, PhD, UCSF professor of obstetrics, gynecology and reproductive sciences.
The team examined a uterus at the proliferative stage of the menstrual cycle, when uterine glands are expanding in preparation for potentially receiving a fertilized embryo, and found that the geometry of the uterine lining in humans appears less convoluted than in mice, but the density and organization of uterine glands in the human uterus appears much more complex. The researchers hope future experiments will reveal whether the human uterus responds to implantation by forming pockets and reorienting the glands, as in the mouse.
In the future, the researchers say, the new 3D imaging approach will be important for understanding and hopefully treating disorders of pregnancy and improving the healthy success rates of implantation of embryos generated by in vitro fertilization, which the authors say is still characterized by a lot of uncertainty.
“This new view of early pregnancy lets us ask fundamentally new questions about how the embryo finds its home within the uterus and what factors are needed for it to implant successfully,” Laird said. “Once we can understand how these processes happen normally, we can also ask why certain genetic mutations cause pregnancies to fail, to study the potential dangers of environmental toxins such as the chemicals in common household products, and even why metabolic disease and obesity appears to compromise implantation.”
“We think these changes in the uterine lining are important for a successful pregnancy,” she added. “How well things work at this earliest stage probably has a lot of effects later on.”
Additional authors on the work were Karina Oelerich and Khalida Sabeur, PhD, of the Edith and Eli Broad Center of Regenerative Medicine and Stem Cell Research at UCSF, and Adam Fries and Kyle Marchuk, PhD, of the UCSF Biological Imaging Development Center.
The research was supported by The California Institute for Regenerative Medicine (TG2-01153), the UCSF Program for Breakthrough Biomedical Research (PBBR) and the National Institutes of Health (5T32HD007263-32, P50HD055764, DP2OD007420 and 1R21ES023297). The authors declare no competing financial interests.
